WARNING: PREP ACT/COVID-19 EUA IMMUNITY IS UNCONSTITUTIONAL AND IMMORAL
Harold Saive
WARNING: PREP ACT/COVID-19 EUA IMMUNITY IS UNCONSTITUTIONAL AND IMMORAL
School Boards believe they are immune to civil and criminal litigation because they are following HHS orders. This email let the San Diego School Board know that they're in for a rude awakening.
May 8, 2023: (Originally published 12/26/2022) In September of 2021, San Unified School District announced they would be voting a mandate to inject all students and faculty ages 16 and older with the COVID-19 injections. I sent this WARNING email to the Board of the San Diego Unified School District (SDUSD) and their Medical Advisory Panel. The Board of the San Diego Unified School District (SDUSD) voted in favor of the mandate, however, my son’s district (which is a subset of SDUSD) announced that they would not be adhering to the Board’s decision.
The SDUSD Board’s decision was eventually struck down by the CA Supreme Court in February of 2023, for many of the reasons I cited in this email, specifically, the Board was exercising authority over the health and safety of the students that they did not legally have.
I will be providing subscribers to The Kingston Report, with another WARNING email soon, on how to DEMAND County Boards and Sheriffs cease the administration of all COVID-19 injections and seize them.
The Kingston Report is reader-supported. To receive new posts, consider becoming a free or paid subscriber.
From: Karen Kingston <***@mifight.com>
Date: Tuesday, September 28, 2021 at 1:34 PM
To: x...@sandi.net , x...@sandi.net,
x...@sandi.net, x...@sandi.net, sch...@ucsd.edu
Cc: x...@foxnews.com
Subject: WARNING: PREP ACT/COVID-19 EUA
IMMUNITY IS UNCONSTITUTIONAL AND IMMORAL
This WARNING is sent to the attention of “Richard Barrera, Sabrina Bazzo, Kevin Beiser, Michael McQuary, and Sharon Whitehurst-Payne,” who are hereafter referred to individually and collectively as the ‘SDUSD BOARD,’ and to, “Richard Garfein, Kimberly Brouwer, Natasha Martin, Robert (Chip) Schooley, Davey Smith, Stephen Spector, and Mark Sawyer,” who are hereafter referred to individually and collectively as the ‘UCSD EXPERT PANEL’.
Per today’s upcoming September 28, 2021, San Diego Unified School District (SDUSD) Board Meeting, the SDUSD BOARD will be voting on the “Vaccination Roadmap” is hereafter referred to as the ‘COVID-19 VACCINATION ROADMAP’. The proposed mandate as it relates to the COVID-19 VACCINATION ROADMAP is referred to as the ‘COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS,’ hereafter.
This communication will focus on the only FDA approved ‘COVID-19 vaccine’ known as COMIRNATY, aka Pfizer/BioNTech mRNA vaccine, for adults and teenagers 16 years of age and older.
As the mother of a teenager who is a student of SMUSD, and a 20+ year biotech/pharma/med-device marketer and analyst, I CONDEMN the COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS.
The SDUSD BOARD’s and UCSD EXPERT PANEL’s authority and influence over the COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS is based on your knowledge, expertise and moral aptitude regarding the health benefits and risks of those students and faculty who may be mandated to be vaccinated. The SDUSD BOARD’s decision to pass or not pass the COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS, as guided by the UCSD EXPERT PANEL, may also be based on the individual and collective knowledge of personal civil and criminal liability risks. To ensure the SDUSD BOARD and UCSD EXPERT PANEL does not have plausible deniability regarding the risk for physical harm, permanent disabilities, autoimmune diseases and deaths for teenagers and young adults aged 16 years of age and older from the COMIRNATY and/or other EUA COIVD-19 vaccines, this communication will focus on some false and misleading statements issued by the SDUSD BOARD and UCSD EXPERT PANEL from the COVID-19 VACCINATION ROADMAP.
It is important to note that NO STATEMENTS MADE by the SDUSD BOARD or UCSD EXPERT PANEL in the COVID-19 VACCINATION ROADMAP provide any scientific, medical, or legal references or source documents. Therefore, it is reasonable to assume that the majority of the scientific, medical, and legal content in the COVID-19 VACCINATION ROADMAP is both false and disingenuous. Per slide 5 of the COVID-19 VACCINATION ROADMAP, Robert (Chip) Schooley, Division of Infectious Disease & Global Public Health, goes so far as to openly confess that his support is also politically motivated when stating, “It (COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS) will also ‘mainstream’ the policy around COVID”.
Per slide 3 of COVID-19 VACCINATION ROADMAP, entitled “The Science,” the first bullet point states:
“Vaccines are fully approved by the FDA only once an extremely high level of confidence that effectiveness and benefits clearly outweigh known or potential risks.”
The above statement referring to COMIRNATY, as well as other FDA authorized vaccines for COVID-19, regarding benefits versus known or potential risks is unfounded, false, and reckless.
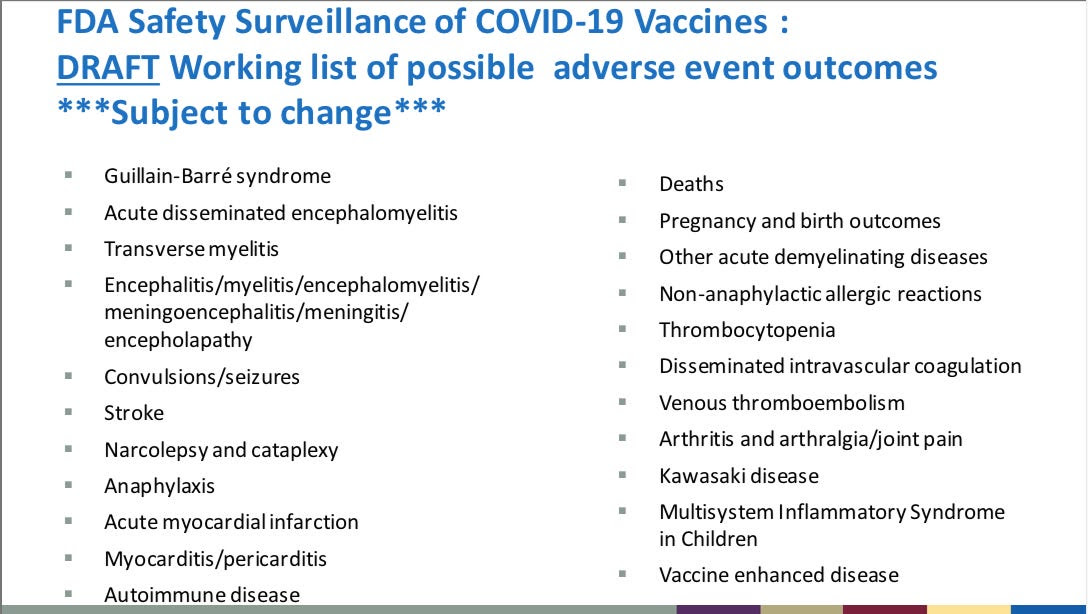
Per the FDA’s own VRBPAC PowerPoint presentation, slide 16, dated October 22, 2020, the FDA states anticipated serious adverse events that can result in permanent injuries, autoimmune diseases, chronic diseases, disabilities, and death from the COVID19 vaccines.
These serious adverse events (SAEs) have been reported in VAERS as well as in the CMS data base. It is important to note that DEATH is listed as a potential adverse event from the COVID-19 vaccines.
Death has proven to be a known adverse event from the COVID-19 vaccines, with 15,386 deaths reported to date in VAERS. In the CMS database 48,465 people died withing 72 hours of their 1st or 2nd dose of a COVID-19 vaccine. This is 3x’s the number of total deaths reported in VAERS. This mortality data is from only up to 3 days post-dose of a 1st or 2nd injection. CMS only accounts for 18% of the US population. As electronic health record (EHR) databases continue to be audited through whistleblowers and lawsuits, the final number of lives lost by the COVID-19 injections will be horrifying to the American people and residents of San Diego County.
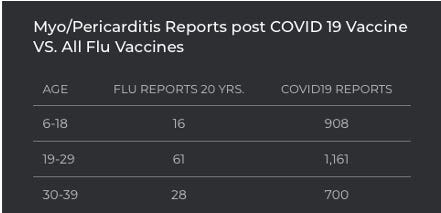
The incidence of myocarditis/pericarditis (heart inflammation) in children and teenagers from the COVID-19 vaccine vs the flu vaccine is alarming. On average, there has been zero to 1 incidence of heart inflammation in children aged 6-18 over the past 20 years of flu vaccines. In less than one year of COVID-19 vaccinations, there are already 908 incidences of heart inflammation reported in VAERS in children alone. The children and young adults who have been admitted to a hospital with heart inflammation are those with severe enough symptoms to merit an emergency visit.
Many children, teenagers, and young adults injected with a COVID-19 vaccine may be suffering more mild symptoms of myocarditis and unaware that their fatigue, weakness, and/or shortness of breath is caused by inflammation of their heart from a COVID-19 vaccine.
Per the FDA’s own approval letter, dated August 23, 2021, there has been enough meaningful incidences of heart inflammation correlated with the Pfizer/BioNTech COVD-19 vaccine to merit SIX (6) additional safety studies regarding the incidence of heart inflammation caused by COMIRNATY and the long-term effects of myocarditis in both adults and children. These myocarditis studies alone should be enough to stop all mandatory vaccinations, as the FDA does not know how quickly this serious adverse event will progress to permanent injuries, chronic diseases, disabilities, and death.
This post is free. Feel free to share!
Per an article in Circulation, the publication of the American Heart Association, prospective data has “implicated myocarditis in sudden cardiac death of young adults at rates of 8.6% to 12%. Furthermore, it has been identified as a cause of dilated cardiomyopathy in 9% of cases in a large prospective series. Recent molecular techniques have facilitated new insights into inflammatory autoimmune processes that affect the myocardium and ultimately result in acute or chronic dilated cardiomyopathy.”
Although the FDA approval letter states that the FDA is unclear regarding the mechanism of action causing heart inflammation, there are several well-established ways the COVID-19 vaccines can cause myocarditis, including but not limited to;
-
The mRNA in the vaccines produces the disease-causing SARS-CoV-2 spike protein that binds to the ACE2 receptors of the heart causing inflammation.
-
The Pfizer lipid nanoparticles (LNPs) that encapsulate the mRNA, have been shown to cause mass inflammation of the heart and lungs in animals.
-
The antibodies produced by the immune system in response to the spike protein from the mRNA vaccine can result in immunogenicity. Immunogenicity can cause autoimmune diseases, such as Pompe’s disease. The first symptom of Pompe’s disease is often heart inflammation and children often have a 2-year prognosis when diagnosed. Please see FDA Presentation – ‘Immunogenicity – What You Don’t Know Can Hurt You and the Patient.’
In case the SDUSD BOARD and EXPERT PANEL are unaware, COMIRNATY is protein-based therapy as it uses synthetic genetic code to produce the SARS-CoV-2 viral spike protein. (See below for verbatim language from pg. 6 of the August 23rd, FDA/BLA approval.)
-
COMIRNATY contains a nucleoside-modified messenger RNA (mRNA) encoding the viral spike glycoprotein (S) of SARS-CoV-2.
Per the FDA’s own expert analysis, entitled Immunogenicity of Protein-Based Therapies, ‘A major problem with protein-based therapeutics is their immunogenicity, that is, their tendency to trigger an unwanted immune response against themselves….antibodies can also cause complications that can be life-threatening.’
While Dr. Fauci has frequently boasted of the mRNA vaccines augmenting a ‘robust immune response,’ in some cases this ‘robust immune response,’ can turn out to be extremely harmful and lethal, especially in children and young healthy teenagers and adults. To further substantiate my previous statement, the FDA and Pfizer have analyzed enough data to determine the extent of the effects of COMIRNATY causing immunogenicity are unknown and that Study C4591007 needs to be completed to ‘evaluate the immunogenicity and safety of lower dose levels of COMIRNATY in individuals 12 through <30 years of age.
Not only is the above listed COMIRNATY immunogenicity study alone enough to make a parent’s heart drop, the safety-study also requires a lower dose of the mRNA that is currently available in COMIRNATY for students 12 years of age and older. The current dose is 30mcg per dose.
Per the FDA’s own documents, it is evident that the known and potential risks of COMIRNATY may result in harm, permanent injuries, autoimmune diseases, and death in SDUSD students and staff, and yet the SDUSD BOARD and EXPERT PANEL have the audacity to proclaim, “that effectiveness and benefits clearly outweigh known or potential risks.”
Furthermore, the statement, “Vaccines are fully approved by the FDA…” is extremely misleading regarding the approval, quality, and availability of COMIRNATY for SDUSD students and faculty aged 16 and older. Although COMIRNATY was FDA approved on August 23, 2021, as of September 28, 2021, many of the Emergency Use Authorization (EUA) Pfizer/BioNTech COVID-19 vials currently available and authorized for injection into children and adults 12 years of age or older are more than likely to be expired, and likely do not contain the same ingredients as the FDA approved mRNA vaccine COMIRNATY.
Even with FDA approval, the ingredients of and the quality, safety and toxicity of the ingredients of the COVID-19 vaccines, including COMIRNATY, are widely unknown by the American public.
EXPIRED AND NON- FDA APPROVED PFIZER/BIONTECH COVID-19 VACCINE VIALS ARE AUTHORIZED FOR INJECTION IN CHILDREN AND ADULTS AGED 12 YEARS AND OLDER
-
Per the August 22, 2021, FDA EUA AMENDMENT issued by Marion Gruber of CBER (Center for Biologics and Research) to Pfizer on behalf of CBER, Ms. Gruber extended the expiration dates of the Pfizer/BioNTEch vaccine lots currently available in the public domain, including Pfizer/BioNTech vaccines available in San Diego, by an additional three (3) months.
-
“We confirm that the concurrence on extending the shelf-life from 6 months to 9 months when stored at -90o to -60oC is also applicable to batches that might have expired prior to the issuance of this concurrence letter provided they have been stored at -90o to -60oC.”
-
-
Under the EUA Guidance, ‘per section 564 of the FD&C Act (Federal Food, Drug and Cosmetic Act), as added by PAHPRA (2013 Pandemic and All-Hazards Preparedness Reauthorization Act)’ the currently FDA approved and available Pfizer/BioNTech mRNA vaccines to SDUSD students and faculty may not have been subject to ‘otherwise-applicable current good manufacturing requirements (cGMP) (e.g. storage and handling)’ that meet the standard cGMP quality and safety guidelines, in order ‘to accommodate emergency response needs.
-
This allows for authorizing expired Pfizer/BioNTech mRNA (COMIRNATY) vaccine for use of injection into students and faculty by extending expiration dates of mRNA vials.
-
MUCH OF PFIZER/BIONTECH COVID-19 mRNA VACCINE’S INGREDIENTS, FORMULATION, QUALITY AND SAFETY ARE UNKNOWN TO THE PUBLIC
-
Per the FDA’s August 23, 2021, Biological License Approval (BLA) approval document based on BioNTech’s (on behalf of Pfizer) Submission entitled, ‘Summary for Regulatory Action’ there are only select lots of EUA Pfizer/BioNTech COVID-19 vaccine vials that are compliant with the FDA approved mRNA vaccine – COMIRNATY - currently available.
-
Health Care Providers have been issued a letter from the FDA (CBER) letting them know that only some lots of EUA mRNA vaccine are compliant with the FDA approved COMIRNATY formulation and the letter instructs the Health Care Providers to a website for additional information (EXHIBIT 5b). Therefore, it is UNKOWN if ANY FDA APPROVED mRNA VACCINES are AVAILABLE in SAN DIEGO to SDUSD students, parents, and faculty.
-
-
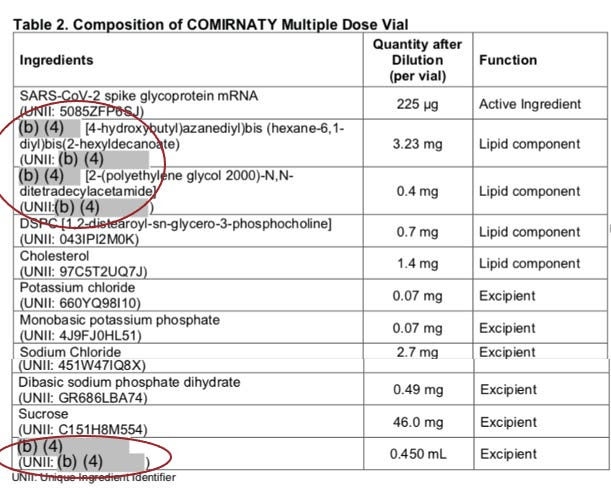
Even with FDA approval, Americans have never received full disclosure of COMIRNATY ingredients, as BioNTech’s (on behalf of Pfizer) submission of ingredients released on August 23, 2021 is partially REDACTED.
-
The quality control tests for the safety and toxicity of COMIRNATY’s ingredients have not been disclosed, as 85% of this information is REDACTED.
-
The EUA letters issued by the FDA on August 23, 2021, and reissued on September 22, 2021 contain FALSE STATEMENTSthat the EUA mRNA Pfizer/BioNtech COVID-19 vaccine is the same formulation and interchangeable with the FDA approved COMIRNATY.
Per slide 3 of the “Vaccination Roadmap,” the SDUSD BOARD and EXPERT PANEL claim, “Vaccine is the most preventive of all strategies.” This statement is proven false by real world evidence presented by the CDC, in an August 2021 MMWR. As mass vaccination of the US Population increases, so does hospitalizations of infants, children and teenagers.
The increase in pediatric hospitalizations, referred to as COVID-19 associated in the above chart, are not likely correlated with natural infection from a natural SARS-CoV-2 virus or its natural variants, but more likely associated with infection from the viral particles of those who are COVID-19 vaccinated. This phenomenon of VGBT injected subjects infecting others is well-documented and known as ‘shedding’ as per the August 2015 FDA document entitled, “Design and Analysis of Shedding Studies for Virus-Based Gene Therapy…” The document also discusses the potential for a virus-based gene therapy (VGBT) to produce a virus that is different from the parent strain (SARS-CoV-2). This phenomena is known as progeny (such as the production of a mutant SARS-CoV-2 strain, such as the potential for Delta to be the progeny of BNT162b2/COMIRNATY).
Evidence that both the FDA and Pfizer are aware of the risks of shedding to the American population, is Study C4591022 from the FDA approval letter, entitled “Pfizer-BioNTech COVID-19 Vaccine Exposure during Pregnancy: A Non-Interventional Post-Approval Safety Study of Pregnancy and Infant Outcomes in the Organization of Teratology Information Specialists (OTIS)/MotherToBaby Pregnancy Registry.”
It's important to note, that both the FDA and Pfizer were aware of the risks of viral shedding and birth defects per pages 67-68, Sec. 8.3.5.1. Exposure During Pregnancy (EDP) of the Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines. EDP occurs if:
-
A female family member or healthcare provider reports that she is pregnant after having been exposed to the study intervention by inhalation or skin contact.
-
A male family member or healthcare provider who has been exposed to the study intervention by inhalation or skin contact then exposes his female partner prior to or around conception.
-
Per the LAST PARAGRAPH Page 68: Miscarriages and spontaneous abortions should be reported. Neonatal deaths that occur within 1 month of birth should be reported, without regard to causality as SAEs. In addition, infant deaths as related or possibly related to exposure to the study intervention.
In addition, COMIRNATY has not proven to be effective in preventing infection with any clinical significance, never mind ‘most effective in prevention.’ In the Phase-3 trials, Vaccine Efficacy (VE) was defined as relative risk of infection and not absolute risk in infection. The absolute risk reduction in infection from COVID-19 was less than a 1% reduction in risk in the COMIRNATY injected group versus placebo. A less than 1% reduction in risk from infection of a virus with an overall 99.9% survival rate, and 99.997% survival rate in children, is of NO CLINICAL SIGNIFICANCE!
Furthermore, per the FDA COMIRNATY September 17, 2021, FDA Briefing Document, page 22 of the document details the results of a post-hoc analysis of the Pfizer Phase-3 trial for COMIRNATY. The analysis indicates:
-
The incidence of COVID-19 increases over time in those subjects who receive 2-doses of COMIRNATY
-
2-doses of COMIRNATY may increase the risk of infection for COVID-19 vs placebo
-
During the first 4 months of Phase 3 = 1.26% of placebo group was infected
-
Over the next 4 months = infection rate increased to 4.34% after the placebo group was injected with COMIRNATY = more than a 300% increase in infection rate!
-
“Although not independently verified by FDA, the post hoc analysis appears to indicate that the incidence of SARS-CoV-2 during the analysis period among 18,727 study participants originally randomized to BNT162b2 (mean of 9.8 months post-Dose 2 at the beginning of the analysis period) was 70.3 cases per 1,000 person-years, compared with an incidence of 51.6 cases per 1,000 person-years among 17,748 study participants originally randomized to placebo and crossed over to BNT162b2 (mean of 4.7 months post-Dose 2 at the beginning of the analysis period). An additional analysis appears to indicate that incidence of COVID-19 generally increased in each group of study participants with increasing time post-Dose 2 at the start of the analysis period. Only 3 severe COVID-19 cases were reported during the analysis period, all of which occurred among study participants originally randomized to BNT162b2.
The reported incidence of COVID-19 among study participants who completed the primary series <4 months prior to the start of the analysis period was 43.4 cases per 1,000 person- years. In contrast, during the blinded, placebo-controlled follow-up period of the study with data cutoff of March 13, 2021 (prior to the Delta variant surge), the incidence of COVID-19 among BNT162b2 recipients in the Evaluable Efficacy Population (nearly 60% of whom had 4 months or more of blinded follow-up post-Dose 2) was 12.6 cases per 1,000 person-years. This observation suggests that while waning immunity is one potential factor that may have contributed to the higher incidence breakthrough cases during the Delta variant surge, it is possible that other factors (e.g., dynamics of Delta variant transmission and potential differences in vaccine effectiveness against the Delta variant vs. strains circulating during the placebo-controlled portion of the trial) may also have contributed.”
I believe one of the major contributing reasons why the SDUSD BOARD and EXPERT PANEL, as well as numerous other local and national organizations are under the delusion that they have the right to yield unbridled tyrannical authority in mandating unhealthy, damaging, and sometimes deadly mandates such as the COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS, is because of the ‘immunity clause’ under the PREP ACT per the EUA.
Willful misconduct is misconduct that is greater than any form of recklessness or negligence. It is defined in the PREP Act as an act or failure to act that is taken:
· intentionally to achieve a wrongful purpose;
· knowingly without legal or factual justification; and
· in disregard of a known or obvious risk that is so great as to make it highly probable that the harm will outweigh the benefit.
All three of these conditions must be proven with clear and convincing evidence.
Willful misconduct cannot be found against:
· A manufacturer or distributor for actions regulated by HHS under the Public Health Service Act or the Federal Food, Drug and Cosmetic Act, if HHS chooses not to take an enforcement action against the manufacturer or distributor, or if HHS terminates or settles an enforcement action without imposing a criminal, civil, or administrative penalty; or
· A program planner or qualified person who acts in accordance with applicable directions, guidelines, or recommendations issued by the HHS regarding administration and use of a countermeasure as long as HHS or the State or local health authority is notified about the serious injury or death within seven days of its discovery.
Unfortunately for the SDUSD BOARD and EXPERT PANEL, the US Constitution and the 14th and 9th Amendments override your delusional authority to place the students and faculty of the San Diego School District at KNOWN RISKS FOR PERMANENT INJURY, AUTOIMMUNE DISEASES, DISABILITIES, and DEATHS with no proven benefits, other than your financial gain. The PREP Act does not override the inalienable God-given rights of the American people granting the SDUSD BOARD and EXPERT PANEL immunity from ‘willful misconduct,’ despite the numerous memos or emails you have received reassuring you that you have malfeasant tyrannical reign to willfully to coerce and conspire to harm, injure, or murder San Diego students and faculty by an FDA approved biological agent.
Under the 9th Amendment, the Federal Government cannot pass laws that enable the government to enable local governments, private organizations, or individuals to commit acts of Domestic violence against U.S. citizens. In other words, for example, conspiracy to commit aggravated assault and murder of minors and adults through coercion cannot be made legal by the US Federal government, CA state elected officials, or other local governments.
COMIRNATY was unlawfully approved by the FDA and has proven to cause PERMANENT INJURY, AUTOIMMUNE DISEASES, DISABILITIES, and DEATHS in children, teenagers, young adults, adults, and the elderly. The long-term risks and mortality are still unknown as the product has not even completed the short-term safety studies of 2-5 years. This is just some of the young lives lost to the COVID-19 injections.
As the mother of a teenager in the SDUSD School District and a 20+ year biotech/pharma/med-device marketer and analyst, I CONDEMN the COVID-19 VACCINE MANDATE FOR SAN DIEGO SCHOOLS.
I have cc’d numerous media outlets, welcoming the SDUSD BOARD and EXPERT PANEL to discuss their decision in the court of public opinion.
Sincerely,
Karen Kingston
San Diego County Resident
The Kingston Report.
TRUTH WINS.
James 2:18
But someone will say, “You have faith, and I have works.” Show me your faith without your works, and I will show you my faith by my works.
See TOWN SQUARE NEWS Updates: https://groups.google.com/g/town-square-news