Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal
chris.vivian2
https://aslopubs.onlinelibrary.wiley.com/doi/10.1002/lol2.10330
Lennart T. Bach, David T. Ho, Philip W. Boyd, Michael D. Tyka
Deliberately altering atmospheric CO2 to influence the Earth's climate has been debated for decades (Keith 2000), but was generally not considered a key strategy for climate mitigation. This viewpoint has changed since The Paris Agreement in 2015, and with the IPCC's 6th assessment report concluding in April 2022 that atmospheric CO2 removal (CDR) is now “unavoidable” to reach net zero emissions (IPCC 2022).
The ocean has great potential to deliver CDR at scale but marine CDR (mCDR) methods have key constraints that differ critically from terrestrial CDR approaches. One of the most pronounced differences is that most mCDR methods create or enhance a seawater pCO2 (partial pressure of CO2) deficit when deployed in regions where the seawater pCO2 is equal to or below atmospheric pCO2. Or, they reduce a seawater pCO2 surplus in regions where seawater pCO2 is higher than atmospheric pCO2 (e.g., Eastern Boundary Upwelling Systems). However, in stark contrast to terrestrial CDR methods, mCDR methods cannot directly remove CO2 from the atmosphere. Atmospheric CO2 removal is only potentially occurring in a subsequent step, namely when surface seawater pCO2 equilibrates with atmospheric pCO2. Thus, atmospheric CO2 removal depends upon how completely atmospheric CO2 equilibrates with the CO2-deficient surface water following an mCDR deployment. Or, when the mCDR deployment is in a pCO2 surplus region, how much of the surplus CO2 would have outgassed into the atmosphere in the absence of the mCDR deployment. The necessary air–sea CO2 exchange depends on various factors such as the residence time of seawater at the surface, carbonate chemistry, and how rapidly atmospheric CO2 invades the surface ocean (Wanninkhof et al. 2009).
Jim Baird
”how much of the surplus CO2 would have outgassed into the atmosphere in the absence of the mCDR deployment”
“Direct Climate Cooling (DCC) can dramatically reduce harm, preserve ecosystems, and save lives as we work to reduce greenhouse gas (GHG) emissions and remove GHG from the atmosphere and oceans,” Healthy Planet Action Coalition.
This cooling can “Ungas” CO2 from the atmosphere.
--
You received this message because you are subscribed to the Google Groups "Carbon Dioxide Removal" group.
To unsubscribe from this group and stop receiving emails from it, send an email to CarbonDioxideRem...@googlegroups.com.
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/8d3cee04-8044-4b16-bc7e-9dc4edc92a7an%40googlegroups.com.
Jim Baird
Hi Ye,
Isn’t that what Resplandy et al. did in Quantification of ocean heat uptake from changes in atmospheric O2 and CO2 composition?
Quantified temperature-dependent fluxes from the various sources and sinks.
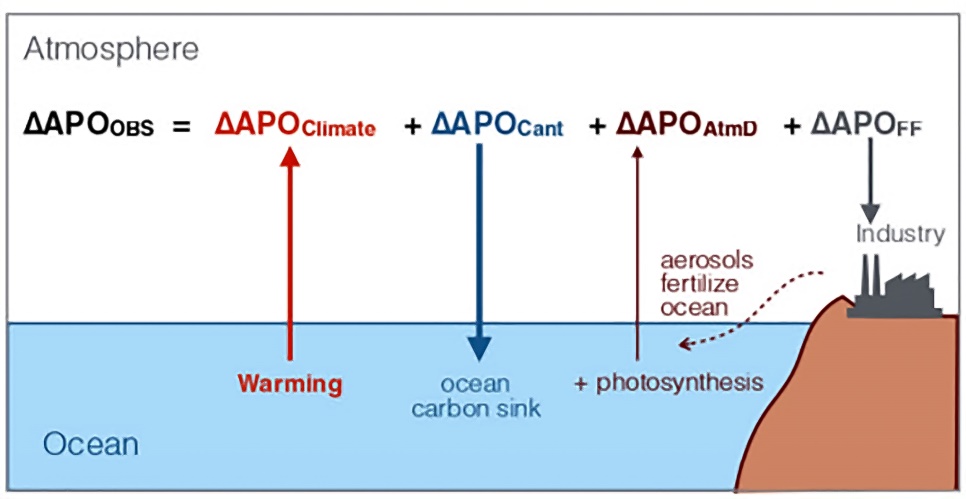
Jim
From: Ye Tao
Sent: May 19, 2023 10:53 AM
To: Jim Baird <jim....@gwmitigation.com>; 'chris.vivian2' <Chris....@btinternet.com>; 'Carbon Dioxide Removal' <CarbonDiox...@googlegroups.com>
Cc: healthy-planet-...@googlegroups.com
Subject: Re: [CDR] Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal
Hi Jim et al,
Can people suggest sources that have quantified temperature-dependent fluxes from the various sources and sinks? Obviously we have the methane (uncertain), permafrost, and forest burning (probably secondary importance wrt fossil fuel burning) emissions at higher temperatures. Can we pool together sources that give the various flux changes vs Del T?
I once believed, simply because many people said it, that the CO2 would outgas from the oceans at higher temperatures. I am not so sure anymore since the ionizations of carbonic acid and the bicarbonate ion seem to be favored by rising temperatures with a gradient proportionally larger compared to that of CO2(g) water solubility decreasing.
Ye
--
You received this message because you are subscribed to the Google Groups "Healthy Planet Action Coalition (HPAC)" group.
To unsubscribe from this group and stop receiving emails from it, send an email to healthy-planet-action...@googlegroups.com.
To view this discussion on the web visit https://groups.google.com/d/msgid/healthy-planet-action-coalition/011401d98a71%2488e3fa80%249aabef80%24%40gwmitigation.com.
For more options, visit https://groups.google.com/d/optout.
Michael Hayes
--
Josh Perfetto
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/CABjtO1edn2Gz5QB4FDTyTV%3DxREQaadgEx7zos5WdZKg9ppyz5Q%40mail.gmail.com.
Michael Hayes
Tom Goreau
Doesn’t the very slow kinetics of CO2 hydration limit the effectiveness of spraying alkaline seawater to absorb CO2?
You may need to add carbonic anhydrase.
Thomas J. F. Goreau, PhD
President, Global Coral Reef Alliance
Chief Scientist, Blue Regeneration SL
President, Biorock Technology Inc.
Technical Advisor, Blue Guardians Programme, SIDS DOCK
37 Pleasant Street, Cambridge, MA 02139
gor...@globalcoral.org
www.globalcoral.org
Skype: tomgoreau
Tel: (1) 617-864-4226 (leave message)
Books:
Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase
http://www.crcpress.com/product/isbn/9781466595392
Innovative Methods of Marine Ecosystem Restoration
http://www.crcpress.com/product/isbn/9781466557734
No one can change the past, everybody can change the future
It’s much later than we think, especially if we don’t think
Those with their heads in the sand will see the light when global warming and sea level rise wash the beach away
Geotherapy: Regenerating ecosystem services to reverse climate change
From: <carbondiox...@googlegroups.com> on behalf of Michael Hayes <electro...@gmail.com>
Date: Saturday, May 20, 2023 at 3:58 AM
To: Josh Perfetto <jo...@snowrise.com>
Cc: Carbon Dioxide Removal <carbondiox...@googlegroups.com>
Subject: Re: [CDR] Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal
Thanks for the interesting questions, Joshs. What I wrote is an early stage idea and questions help me work out the details. The broader concept that I'm working on, beyond misting alkiline water, involves a rather large basket of technical systems being coupled together for both mCDR and oceanic farming, possibly resulting in C negative self sustaining offshore human colonies.
To view this discussion on the web visit
https://groups.google.com/d/msgid/CarbonDioxideRemoval/CABjtO1fi%3D2gUKkVGFyNX%3Dcn0P2m5Q24fTFBV1WxXyQ8doaC%2BZQ%40mail.gmail.com.
Michael Hayes
Jim Baird
Ye, this is how I tried suss out C fluxes as a function of T from this paper.
They determined about 1.11 ± 0.68 per meg or parts per million (ppm) of O2 and CO2 is going into the atmosphere on account of warming of the tropical surface, with the concentrations of these gases being 1 part O2 to 1.05 parts CO2.
About .56 ppm of CO2 was therefore added to the atmosphere each year of the study due to warming.
The atmospheric CO2 level as of Aug. 22, 2021 was 414.68 ppm and Aug. 22, 2020 it was 412.68 ppm so, the annual accumulation was 2 ppm. Since 1 ppm CO2 = 7.81 gigatonnes (Gt), about 15.6 Gt of the greenhouse gas went into the atmosphere over the course of the year, with the 28% of this due to surface warming, which amounts to 4.4 Gt.
They said the oceans gained 1.29 ± 0.79 × 1022 Joules of heat per year between 1991 and 2016 doesn’t this give you the same result as Temperature?
From: Ye Tao
Sent: May 21, 2023 12:37 PM
To: Jim Baird <jim....@gwmitigation.com>; 'chris.vivian2' <Chris....@btinternet.com>; 'Carbon Dioxide Removal' <CarbonDiox...@googlegroups.com>
Cc: healthy-planet-...@googlegroups.com
Subject: Re: [CDR] Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal
Hi Jim,
Thanks for the paper. I think not quite what I was looking for. I am looking for changes to C fluxes as a function of T as the independent variable. The paper you suggest is looking the otherway, and aggregates all sources and sinks wrt the atmosphere.
Ye
To view this discussion on the web visit https://groups.google.com/d/msgid/healthy-planet-action-coalition/014701d98a7c%24313e8920%2493bb9b60%24%40gwmitigation.com.
Jim Baird
Hi Ye,
I agree. Attached is a link to a proposed closed experiment that could test if carbon dioxide moves from the atmosphere into the ocean as the atmosphere is cooled http://gwmitigation.com/Videos/TG5minladderpiitch.m4v.
The video is dated because I would now use 3D printed, thin film, heat exchangers.
According to CleanTechnica there are .75 grams of CO2 in a cubic meter of atmosphere.
The atmosphere in the experiment is 14 cubic meters so there would be 10.5 grams of CO2 and the atmosphere would be additionally at 30C.
The system should be able to power 3 - 100 watt light bulbs. Obviously it couldn’t do this without an external source of power but the point is, producing this energy causes surface heat to move into the cold water sink. So, the experiment would run at a surface temperature of 30C for 6 months and then drop the surface temp by 1.8C – about how much the surface will have reached in about 30 years, and see how much efficiency you lose by cooling the surface and cooling the deep water. In real world conditions a 1000 meter long column of water should be warmed by less than .2C. After 6 months at the cooler surface there should be a migration of CO2 from the atmosphere into the water. According to Resplandy this should be 28% of the 10.5 grams.
Completing my original thought, the ocean contains 1.347e18 cubic meters of water.
The specific heat of water is 4,184 Joules per kilogram.
It would take 4,184 * 1.347e18 cubic meters = 5.57e22 joules to warm the ocean 1 degree.
Resplandy calculated the ocean warmed by 1.29 ± 0.79e22 each year between 1991 and 2016, which would be .23 degrees?
Since this can never be tested at a global scale, this small closed system experiment could give us a clue?
Jim
From: Ye Tao
Sent: May 22, 2023 7:09 AM
To: Jim Baird <jim....@gwmitigation.com>; 'chris.vivian2' <Chris....@btinternet.com>; 'Carbon Dioxide Removal' <CarbonDiox...@googlegroups.com>
Cc: healthy-planet-...@googlegroups.com
Subject: Re: [CDR] Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal
Hi Jim,
I don't think one can get at the fluxes by involking historical CO2(atm) changes, with historical temperature changes and heat accumulation.
These carbon fluxes should be "measured" with anthropogenic fluxes turned down to zero, while temperature is changed by an extraterrestrial source that is not connected to the various forcings involving carbon based gases. Obviously, it is not possible to do such measurements from a top-down approach, so results necessarily need to emerge from small scale mechanistic experiments extrapolated to the global scale.
Ye
Jim Baird
Correction
4,184 * 1.347e18 cubic meters = 5.57e21 joules so the ocean would have warmed by 2.3 degrees which is certainly not the case.
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/004701d98cc8%24a91a9620%24fb4fc260%24%40gwmitigation.com.
