Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal
Geoengineering News
Tom Goreau
This is of course the well-known Biorock process with a newly made up name, but as typical, does not cite any of the many publications on it since 1979!
Thomas J. F. Goreau, PhD
President, Global Coral Reef Alliance
Chief Scientist, Blue Regeneration SL
President, Biorock Technology Inc.
Technical Advisor, Blue Guardians Programme, SIDS DOCK
37 Pleasant Street, Cambridge, MA 02139
gor...@globalcoral.org
www.globalcoral.org
Skype: tomgoreau
Tel: (1) 617-864-4226 (leave message)
Books:
Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase
http://www.crcpress.com/product/isbn/9781466595392
Innovative Methods of Marine Ecosystem Restoration
http://www.crcpress.com/product/isbn/9781466557734
No one can change the past, everybody can change the future
It’s much later than we think, especially if we don’t think
Those with their heads in the sand will see the light when global warming and sea level rise wash the beach away
Geotherapy: Regenerating ecosystem services to reverse climate change
--
You received this message because you are subscribed to the Google Groups "Carbon Dioxide Removal" group.
To unsubscribe from this group and stop receiving emails from it, send an email to
CarbonDioxideRem...@googlegroups.com.
To view this discussion on the web visit
https://groups.google.com/d/msgid/CarbonDioxideRemoval/CAHJsh99gOzxNFrFGt8vp5WUJwxEtoUq4%2BNDxu%3Db4v1iC%3DaK9xQ%40mail.gmail.com.
Chris Van Arsdale
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/A7082462-C19E-4744-9616-29E45CD4B384%40globalcoral.org.
Michael Hayes
Michael Hayes
Michael Hayes
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/A7082462-C19E-4744-9616-29E45CD4B384%40globalcoral.org.
Stefano Caserini
Tomorrow Monday 8th and Tuesday 9th afternoon in Milan (Politecnico di Milano e Università Milano Bicocca) there will be the conference “CO2 sequestration in seawaters: motivation, opportunities and methods”
The language of the conference will be Italian.
Here is the detailed program https://indico.chem.polimi.it/event/65/attachments/147/347/Oceano%20Amico_Locandina.pdf
The link to register for the web streaming of the conference is here https://indico.chem.polimi.it/event/65/registrations/107/
The main topics of the conference will be OAE (ocean alkalinity enhancement), BAWL (buffered accelerated weathering of limestone), and Limenet (an evolution of BAWL, https://limenet.tech/en/).
Best regards,
Stefano
Ing. Stefano Caserini, PhD
Adjunct professor of Mitigation of climate change
Project Manager Desarc-Maresanus Project
Politecnico di Milano, DICA - Dipartimento di Ingegneria Civile ed Ambientale, Sez. Ambientale.
Via Golgi 39, 20133 Milano (Italia)
tel. +39 02 23996414; +39 3289651530
email: stefano....@polimi.it
Ken Caldeira
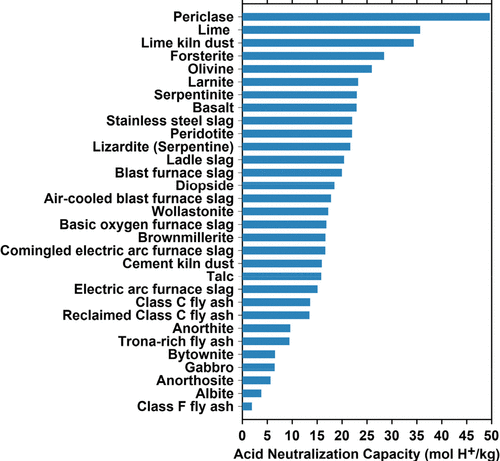
Isn't the main challenge finding an appropriate mineral source for cations such as Ca2+ or Mg2+?
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/CABjtO1e2VS%2B97KBw3uX3_94dQmeqy6x75jxrD%3DLAA7hD5QtG6Q%40mail.gmail.com.
Michael Hayes
Tom Goreau
We work directly in open seawater.
Many more complicated versions can be done by separating reactions and adding exotic materials, but they will impose costs and lower efficiency.
Ken Caldeira
In the absence of alkalinity addition, isn't carbonate formation a source of CO2?
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/A93F3B40-EE8B-4A47-A4B3-3E7DA0026A1C%40globalcoral.org.
Tom Goreau
Yes, that’s true at pH 8, but the pH on the electrode where brucite precipitation happens is so high above the carbonic acid second dissociation constant pK2 that bicarbonate ion is converted to carbonate ion, and the precipitation reactions become:
Mg++ + 2OH- Mg(OH)2
Ca++ + CO3= CaCO3 (without generating CO2, unlike the reaction with bicarbonate)
Brucite dissolves below pH 11-12, releasing two hydroxyl ions which raise the pH and react with Ca++ and either HCO3- or CO3= depending on local pH precipitating aragonite that replaces brucite. The same happens at the black smoker deep sea vents where brucite becomes replaced by aragonite as pH falls.
In addition the net Biorock reaction is alkalinizing because any electrons that react with chloride do not generate hydrogen ions like those that react with water, so seawater electrolysis acts on a local scale to reverse ocean acidification, and the carbonate produced is CO2 negative!
Michael Tyka
Yes, that’s true at pH 8, but the pH on the electrode where brucite precipitation happens is so high above the carbonic acid second dissociation constant pK2 that bicarbonate ion is converted to carbonate ion, and the precipitation reactions become:
Mg++ + 2OH- Mg(OH)2
Ca++ + CO3= CaCO3 (without generating CO2, unlike the reaction with bicarbonate)
Brucite dissolves below pH 11-12, releasing two hydroxyl ions
Michael Hayes
Tom Goreau
Thanks for these comments. You can’t consider electrode half reactions separately and we don’t!
The primary reaction is water hydrolysis, which produces equal amount of hydroxyl and hydrogen ions at opposite electrodes. The hydroxyl does not increase alkalinity in the water, even though many are apparently assuming that, it is very rapidly neutralized on the electrode surface to form brucite and aragonite. There is no measurable increase in ocean water pH even right next to the minerals on the cathode. The anode hydrogen ion production is neutralized by reaction with limestone sediments, but more slowly. If water hydrolysis alone takes place, the limestone deposition is CO2 neutral, in effect transferring limestone from sediment into massive reef structures that regenerate marine ecosystems, biomass, sediment carbon, and far more biological limestone production than is produced by electrolysis.
The key reaction at the cathode (which you don’t list) is OH- + HCO3- CO3= + H2O, which does not generate CO2
Some electrons react with chloride at the anode to form chlorine, but that reaction produces no acidity, so to the extent that it occurs, the mineral production is carbon negative. The Nernst Equation shows oxygen is the main pathway due to the lower redox potential of the half reaction that produces it versus that for chlorine production, and the vastly higher concentration of water than chloride.
Our direct observations underwater on around 600-700 such reefs around the world is that whatever chlorine is produced at the anode reacts rapidly as an oxidant in sea water and is very quickly neutralized. I can’t see any effect on coral tissue further than a millimeter from the anode, and fish swim happily over it, they seem to benefit from the oxygen and don’t avoid what chlorine is produced because it is apparently too dilute to bother them! We are NOT pumping toxic clouds of chlorine into the stratosphere, as you say!
We get huge biological and structural benefits creating wave resistant growing reefs full of corals, oysters, and fishes that keep up with sea level rise with negligible negative biological effects, and generate large amounts of limestone sand from accelerated growth of coralline algae. We greatly increase biological organic carbon production by seagrasses and saltmarshes and their sediment storage, a major carbon sink. We are also producing large amounts of hydrogen, which fuels hydrogen-oxidizing autotrophs and nitrogen fixing microbes, and which produces green hydrogen if solar or wind power is used, plus oxygen that the ecosystems need.
It is clear that accurate measurements of ALL of the products at BOTH electrodes under field operating conditions is needed to understand the net balances.
This one and a half minute video of seagrass, coral, and fish regeneration in the Bahamas, shows that the zone affected by the anode (which is inside the structure) is negligible, there is NO huge dead zone poisoned by chlorine as you suggest must take place:
Thomas J. F. Goreau, PhD
President, Global Coral Reef Alliance
Chief Scientist, Blue Regeneration SL
President, Biorock Technology Inc.
Technical Advisor, Blue Guardians Programme, SIDS DOCK
37 Pleasant Street, Cambridge, MA 02139
gor...@globalcoral.org
www.globalcoral.org
Skype: tomgoreau
Tel: (1) 617-864-4226 (leave message)
Books:
Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase
http://www.crcpress.com/product/isbn/9781466595392
Innovative Methods of Marine Ecosystem Restoration
http://www.crcpress.com/product/isbn/9781466557734
No one can change the past, everybody can change the future
It’s much later than we think, especially if we don’t think
Those with their heads in the sand will see the light when global warming and sea level rise wash the beach away
Geotherapy: Regenerating ecosystem services to reverse climate change
From: Michael Tyka <mike...@gmail.com>
Date: Sunday, May 7, 2023 at 11:36 PM
To: Tom Goreau <gor...@globalcoral.org>
Cc: "kcal...@gmail.com" <kcal...@gmail.com>, Michael Hayes <electro...@gmail.com>, Andrew Lockley <andrew....@gmail.com>, Carbon Dioxide Removal <carbondiox...@googlegroups.com>
Subject: Re: [CDR] Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal
Yes, that’s true at pH 8, but the pH on the electrode where brucite precipitation happens is so high above the carbonic acid second dissociation constant pK2 that bicarbonate ion is converted to carbonate ion, and the precipitation reactions become:
Mg++ + 2OH- è Mg(OH)2
Without an external source of alkalinity where do the 2OH- come from ?
They have to come from water dissociation: H2O --> H+ + OH-
Those H+ then react with HCO3- to make CO2 and H2O:
2H+ + 2HCO3- --> 2CO2(g) + 2H2O, making the net reaction:
Mg2+ + 2HCO3- --> 2CO2(g) + Mg(OH)2
Now perhaps the OH- was formed on a cathode, turning those H+ into H2 gas:
2H2O + 2e- --> H2 + 2OH- Great, no H+ !
But if there's a cathode, there's gonna be an anode somewhere (those 2e- need to come from somewhere): There we can either oxidise chloride ions (making chlorine gas (yuck!)) or we can oxidize H2O, which unfortunately once again makes H+: H2O --> 2H+ + 2e- + 0.5O2
Ca++ + CO3= è CaCO3 (without generating CO2, unlike the reaction with bicarbonate)
Likewise, where does the CO3 come from ? It'd come from the bulk HCO3- in the bulk water, e.g.
2HCO3- --> CO2(g) + CO3(2-)
So we have the same issue, CO2 is released.
Brucite dissolves below pH 11-12, releasing two hydroxyl ions
Unless the brucite comes from land, those two hydroxyl ions earlier released two H+ ions somewhere else.
In addition the net Biorock reaction is alkalinizing because any electrons that react with chloride do not generate hydrogen ions like those that react with water,
Michael Tyka
Some electrons react with chloride at the anode to form chlorine, but that reaction produces no acidity, so to the extent that it occurs, the mineral production is carbon negative.
whatever chlorine is produced at the anode reacts rapidly as an oxidant in sea water and is very quickly neutralized.
Tom Goreau
Thanks for your comments Mike!
We can certainly imagine many potential chemical reactions, some of which may be thermodynamically feasible but kinetically inhibited (like calcite precipitation or CO2 hydrolysis to carbonic acid, which organisms bypass with carbonic anhydrase, one of the most abundant enzymes in both plants and animals because it accelerates this reaction and plays a key role in regulating tissue pH and photosynthesis), and there are far more heterogeneous reactions on organic and mineral surfaces!
A more serious unknown is the kinetics of electrolysis overpotential reactions, about which I understand almost nothing (but others certainly do, there are many great electrochemists out there!). What we need is to measure what really happens under natural conditions to understand the net balances and impacts. Variations on the reaction, such as using salt bridges or membranes to separate electrodes into compartments and adding exotic chemicals to each, are readily done (and proposed by many with interesting possibilities). Most of these folks are motivated by monetary reward$, we’re just trying to regenerate dying ecosystems and their essential services while storing their carbon. There is no funding for that at all!
As far as truly carbon-negative Biorock building material grown in the sea goes, we grow brucite powder in the sea, grind it in mortar and pestle, and use that as a wet paste cement that sets solid by absorbing CO2 directly from the atmosphere to make magnesite (MgCO3), which is harder than calcite or aragonite, and 52.4% CO2 by weight:
T. J. Goreau, 2012, Marine electrolysis for building materials and environmental restoration, p. 273-290 in Electrolysis, J. Kleperis & V. Linkov (Eds.), InTech Publishing, Rijeka, Croatia
We made bricks out of this in Jamaica more than 30 years ago. Biorock cement material is harder than Portland Cement and much cheaper to produce with current solar electric generation prices. Coastal countries, especially Small Island Developing States (SIDS) like mine where this was first done, could use it to grow harder genuinely carbon-negative building material in the sea, save a fortune on Portland Cement imports, remove CO2 directly from the air itself (the opposite of Portland Cement), making green hydrogen, and protect their coasts from sea level rise that won’t go away for thousands of years. Nobody is helping SIDS develop their own technology to save themselves, they are more interested in copying it, rebottling it under new names, and selling it back to them for inve$tor$!
It’s important to note that although there is a lot of bicarbonate in the water, it is highly pH buffered, and very slow to turn over: 100,000 years! Changing ocean alkalinity will take a long time to change CO2 in the atmosphere by equilibrium reactions. You can do that faster with land biomass, more permanently with blue carbon (sea grass, salt marsh, and mangroves), and most permanently by turning into Biorock building material that takes CO2 from the air and can last hundreds of millions of years until it goes down a subduction zone.
Thomas J. F. Goreau, PhD
President, Global Coral Reef Alliance
Chief Scientist, Blue Regeneration SL
President, Biorock Technology Inc.
Technical Advisor, Blue Guardians Programme, SIDS DOCK
37 Pleasant Street, Cambridge, MA 02139
gor...@globalcoral.org
www.globalcoral.org
Skype: tomgoreau
Tel: (1) 617-864-4226 (leave message)
Books:
Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase
http://www.crcpress.com/product/isbn/9781466595392
Innovative Methods of Marine Ecosystem Restoration
http://www.crcpress.com/product/isbn/9781466557734
No one can change the past, everybody can change the future
It’s much later than we think, especially if we don’t think
Those with their heads in the sand will see the light when global warming and sea level rise wash the beach away
Geotherapy: Regenerating ecosystem services to reverse climate change
From: Michael Tyka <mike...@gmail.com>
Date: Monday, May 8, 2023 at 1:19 PM
To: Tom Goreau <gor...@globalcoral.org>
Cc: "kcal...@gmail.com" <kcal...@gmail.com>, Michael Hayes <electro...@gmail.com>, Andrew Lockley <andrew....@gmail.com>, Carbon Dioxide Removal <carbondiox...@googlegroups.com>
Subject: Re: [CDR] Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal
Hi Tom,
John Macdonald
On 9 May 2023, at 4:04 am, Tom Goreau <gor...@globalcoral.org> wrote:
--
You received this message because you are subscribed to the Google Groups "Carbon Dioxide Removal" group.
To unsubscribe from this group and stop receiving emails from it, send an email to CarbonDioxideRem...@googlegroups.com.
To view this discussion on the web visit https://groups.google.com/d/msgid/CarbonDioxideRemoval/6DC75C37-AB4C-4F73-B876-20A336045234%40globalcoral.org.
Toby Bryce
(Dec 2021 Ep16 with SeaChange here.)
